Which Statement Best Describes the Ph of Pure Water
It is said that there is no life without water. Anything higher than 7 would make water acidic already.
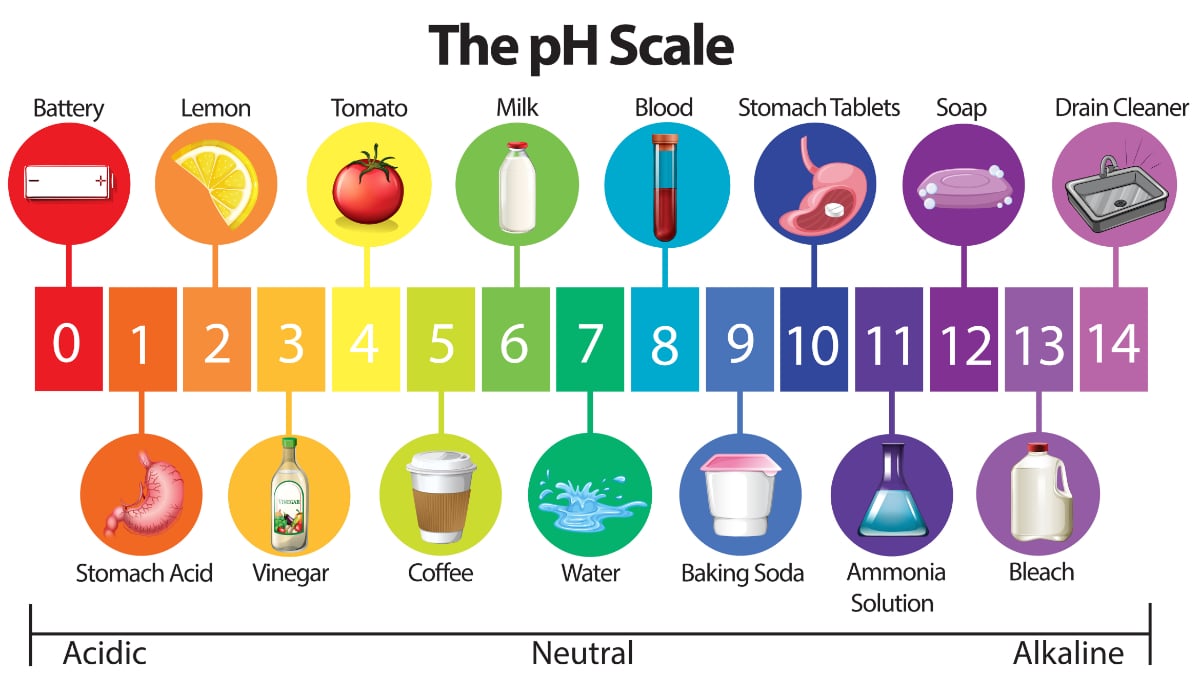
Water Quality 101 What Is Ph In Water Testing
Purified water that has very few ionic species is said to be low in alkalinity ionic strength.

. Gravity Which statement best describes the pH of pure water. It is acidic because it has a hydronium ion concentration of 10 x 10-7 M. The pH of pure water has been best described as neutral pH with equal hydronium and hydroxide ions.
Use the reaction to answer the question. The answer is letter A. The answer is letter A.
Chemistry 22062019 0730 10040813. O H2O is an acid and Cl- is its conjugate base. Rainwater pOH 85 2.
It is neutral because the concentration of hydronium ions equals that of hydroxide ions. PH has been described as the measurement of hydrogen ions in a solution. Click card to see definition It is neutral because the concentration of hydronium ions equals that.
What is the equilibrium constant of pure water at 25C. It is basic because it has a hydroxide ion. Pure water has a 614 pH in temperatures that are at 100 degrees celsius.
It is neutral because the concentration of hydronium ions equals that of hydroxide ions b. It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions. Cola pOH 11 3.
The pH of water is 7 which is equal to 10-7 of H and OH- ions. The pH of high purity water is generally in the range of 55 to 75 depending on the level of carbon dioxide CO2 in the water. Answer Expert Verified 10 5 5 Hagrid Pure water has a 614 pH in temperatures that are at 100 degrees celsius.
The pH of pure water is neutral because the concentration of hydronium ions equals that of hydroxide ions. It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions. The pH of water is 7 which is equal to 10-7 of H and OH- ions.
It is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions. Which statement best describes the pH of pure water a. Thus option Ais correct.
Lake water pH 65 3. 4 Which statement best describes the pH of pure water. It is neutral because the concentration of hydronium ions equals that of hydroxide ions.
ASHA 777 7 1 year ago. Water is one of the most important constituents of living being. Basic Identify each of these substances as acidic basic or neutral.
Baking soda solution pH 9 4. Which statement best describes the pH of pure water. This just means that pure water is in the neutral zone of the pH scale.
Tomato juice pOH 10 4. Which statement best describes the pH of pure water. This just means that pure water is in the neutral zone of the pH scale.
It is acidic because it has a hydronium ion concentration of mc027-1jpg. PH is equal to the negative log of hydrogen. Sun Mar 20 2016 The statement that best describes the pH of pure water is it is neutral because the concentration of hydronium ions equals that of hydroxide ions.
It is neutral because the concentration of hydronium ions equals that of hydroxide ions. Which statement best describes the pH of pure water. O Acids are substances that contain more protons than bases.
Advertisement Hagrid The statement that best describes the pH of pure water is it is neutral because the concentration of hydronium ions equals that of hydroxide ions. Liquid drain cleaner pOH 0 1. So that water need to neutral in nature to save life.
If water reaches a 7 pH scale it is considered as an alkaline water. The pH has been measured on a scale of 1-14pH 7 has been the neutral pH. A solution that has a pH of 2 could best be described as being ________.
It is acidic because it has a hydronium ion concentration of mc027-1jpg. By subtracting the pOH from 14 What is. Advertisement Answer 50 5 0 mcannady4.
Low conductivity water is described by ASTM D5464 as water with a conductivity of 100 μScm. Comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. It is neutral because the pure liquid contains neither hydronium ions not hydroxide ions c.
The table compares the number of electrons in two unknown neutral atoms. HCI H2O Cl H30 Which statement about the reaction is correct. An increased rate of breathing as a result of an increased buildup of carbon dioxide in the bloodstream would be best described as an example of ________.
Excreation of metabolic waste. If water reaches a 7 pH scale it is considered as an alkaline water. 1 point O HCl is an acid and Cl- is its conjugate base.
CIt is acidic because it has a hydronium ion concentration of mc027-1jpg. Which statement best describes the pH of pure water. Pure water pH 70 2.
AIt is neutral because the concentration of hydronium ions equals that of hydroxide ions. It is acidic because it has a hydronium ion concentration of 10 times 10 to the negative 7 moles per liter. BIt is neutral because the pure liquid contains neither hydronium ions nor hydroxide ions.
Which statement best describes ph of pure water Other questions on the subject. The cavities housing the eyes are called __________ cavities. Soapy water pH 12 1.
A low OH- and a high pOH. The higher hydronium ion concentration tends to move the pH from 7 towards 1. PH is equal to the negative log of hydrogen.
O Acids are substances that contain fewer protons than bases. How can the pH of a solution be calculated if the pOH is known. It is neutral because the concentration of hydronium ions equals that of hydroxide ions.
Which statement best describes the pH of pure water. It is neutral because the concentration of hydronium ions equals that of hy droxide ions. Which statement best describes the ph of pure water.
A substance with a high H would likely have which additional characteristics.

I Just Want To Spend Every Possible Minute Of The Rest Of My Life With You Life Love Life Of My Life


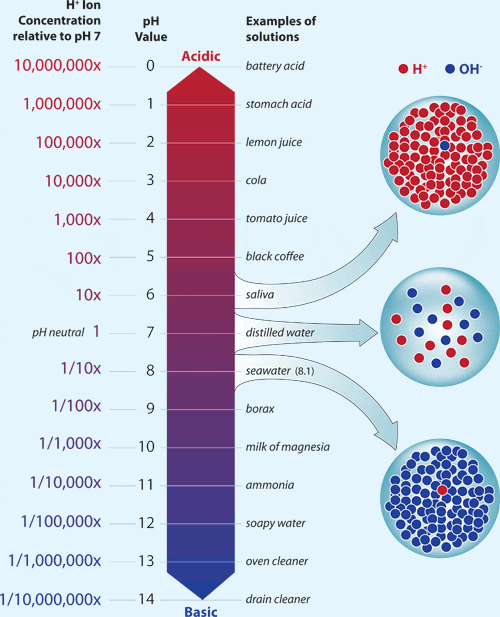
0 Response to "Which Statement Best Describes the Ph of Pure Water"
Post a Comment